THE "FATAL FLAWS" WITH RICHARD CARRIER'S RNA SELF-ASSEMBLY EXAMPLES
The "Fatal Flaws" with Richard Carrier's
RNA Self-Assembly Examples
Richard Carrier’s claim:
"Another error Totani makes having the same effect relates to a different problem. Totani mentions that we have already discovered actual self-replicating RNA sequences between 100 and 150 nucleotides (Wachowiusa & Holliger 2019; Horning & Joyce 2016); but these have been shown to self-assemble in natural conducive environments from smaller sequences. So they did not have to arrive purely at random as Totani assumes..."
"Even if we were to estimate the probability of a single specific one, we still end up in the realm of accessible probability. For example, Wachowiusa & Holliger 2019 took a self-replicator of 150 nucleotides and broke it up into shorter strands of 20 or 30 nucleotides each and showed a self-replicating 150 nucleotide strand forms inevitably from them in the right context at a measurable rate (countless copies spontaneously formed). Across all volumes in the universe of randomly chaining nucleotides, there will form 10^120 more chains of 30 nucleotide length than of 150 nucleotide length. That’s a one followed by one hundred and twenty zeroes. The probability that several such chains of the right sequence will form in the same volume and then link together, by that inevitable process, into a 150-nucleotide self-replicator is vastly greater than the probability of a spontaneous random sequence of 150 nucleotides forming by itself. As the probability of their so chaining once available is effectively 100% in the specified environ, the total probability of such an assembly approaches that of the spontaneous assembly of molecules 40-60 nucleotides long."
Q's Verdict:
Richard misrepresents Totani and these two studies, which are proof of concept/principle lab-designed RNA replicases that are not even self-replicators, nor do they automatically "self-assemble."
Here's what Totani (2020) actually says in his "Emergence of Life in an Inflationary Universe":
"A key quantity is the minimum RNA length required to show a self-replicating ability. RNA molecules shorter than 25 nucleotides (nt) do not show a specified function, but there is a reasonable hope to find a functioning replicase ribozyme longer than 40–60 nt8,9. RNA polymerase ribozymes produced by in vitro experiments so far have a length longer than 100 nt10,11,12. Furthermore, formation of just a single long strand may not be sufficient to initiate an abiogenesis event. Instead a pair of identical strands may be necessary if one serves as a replicase ribozyme and the other as a template." (emphasis added)."
Horning & Joyce (2016), and Wachowiusa & Hollinger (2019) are Totani's reference 11 & 12, respectively, in the quote above. Thus, we see that Totani does not say "we have already discovered actual self-replicating RNA sequences between 100 and 150 nucleotides"; but that "RNA polymerase ribozymes"—which are not self-replicating—have been "produced by in vitro experiments"—not "discovered"—"longer than 100 nt"—not "between 100 and 150 nucleotides."
Horning & Joyce (2016):
Source citation: Horning, D. P., & Joyce, G. F. (2016). Amplification of RNA by an RNA polymerase ribozyme. Proceedings of the National Academy of Sciences, 113(35), 9786-9791.
Summary: This is another proof of concept/principle study concerning a lab-designed RNA polymerase ribozyme 180 nucleotides long (not "between 100 and 150 nucleotides") that is not self-replicating (nor does it "self-assemble); which demonstrates that "the replication of RNA and the expression of functional RNA can be accomplished with RNA alone [without proteins]." As such, it is considered too complex to spontaneously emerge, and could only serve as a replicase since it cannot self-replicate; which would require a second RNA template strand as Totani notes: "Furthermore, formation of just a single long strand may not be sufficient to initiate an abiogenesis event. Instead a pair of identical strands may be necessary if one serves as a replicase ribozyme and the other as a template." (emphasis added)."
 |
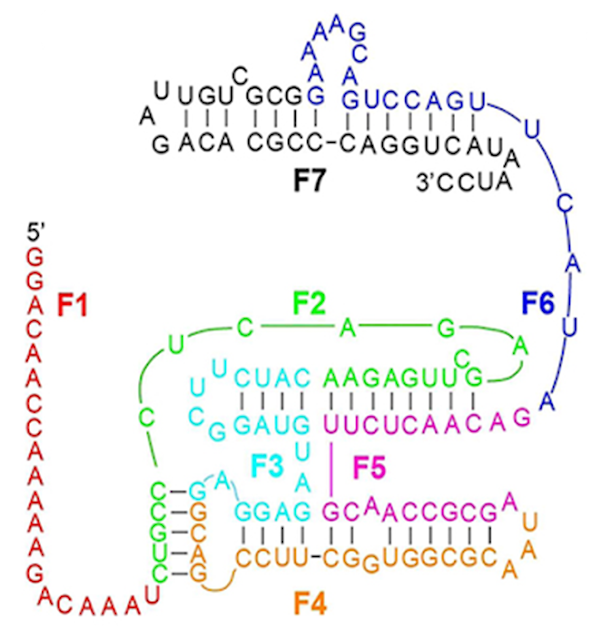
| Fig. 1C: "Sequence and secondary structure of the [180nt] 'polymerase 24-3'.'" Horning & Joyce (2016) |
Wachowiusa & Hollinger (2019):
Source citation: Wachowius, D., & Holliger, P. (2019). Non-enzymatic assembly of a minimized RNA polymerase ribozyme. ChemSystemsChem, 1(1-2), 1.
Summary: This is not a self-replicating RNA! Nor does this 150 nucleotide RNA polymerase ribozyme "assemble automatically" from seven shorter RNA strands, as Richard erroneously states. Six additional RNA strands that serve as scaffolding "splints" are required to correctly sequence and assemble the 150nt ribozyme (and even then it almost never works: a paltry 0.5% yield!). In other words, thirteen RNA strands totaling 280 nucleotides are needed to assemble this 150 nucleotide ribozyme (almost twice as many specifically sequenced nucleotides!); giving (on paper) 1 chance in 4^280 = 1 chance in 10^168, which is not expected to spontaneously occur based on Richard's 1 in 10^150 standard. Once again, Richard has not carefully read or understood the research he cites. This is another "proof of concept" study that does not claim to be prebiotically plausible, nor is it prebiotically plausible, nor is it even a self-replicating RNA!
Richard writes: “Another error Totani makes having the same effect relates to a different problem. Totani mentions that we have already discovered actual self-replicating RNA sequences between 100 and 150 nucleotides (Wachowiusa & Holliger 2019; Horning & Joyce 2016); but these have been shown to self-assemble in natural conducive environments from smaller sequences. So they did not have to arrive purely at random as Totani assumes; a common mistake I identified under peer review as a Class VII error, and discuss in my analysis of Totani: evidence (not speculation) proves that some aspects of the first protobiont don’t require purely random assembly, but assemble automatically, with a probability in the described environments of effectively 100%.”
But here, once again, Richard is flat out wrong. They have not been shown to “self-assemble in natural conducive environments from smaller sequences,” nor “assemble automatically”! Richard has committed numerous errors of his own. Let’s break this down for the Wachowiusa & Holliger 2019 example Richard specifically uses of a 150 nucleotide “self-replicator” “self-assembled” from 7 shorter pieces to illustrate his claim.
1. First, the 150 nucleotide "self-replicator" in Wachowiusa & Hollinger (2019) was not a "self-replicator" (i.e., it doesn't replicate itself), but was a lab-engineered "minimal RNA polymerase ribozyme variant." And they did not simply "[break] it up into shorter strands...and [show] a self-replicating 150 nucleotide strand forms inevitably from them." It is substantially more complicated than that. Richard makes it sound like these shorter strands are like dispersed oil droplets that automatically self-assemble/merge under thermodynamic control to form a larger oil drop. That picture is entirely false and misleading. The picture he paints of “automatic” “self-assembly” in a “natural environment” is wrong on all three counts (as discussed below).
2. Second, purified reagents were used, including purified stocks of the shorter 7 oligonucleotide pieces (See, Supplementary Information, Wachowiusa & Hollinger 2019), which is not a realistic simulation of the "messy chemistry" that would occur in prebiotic environments. The use of pure reagents is not “prebiotically plausible” (See below, Bains 2020). Synthesis also occurred under controlled laboratory conditions that controlled for pH, temperature, salt concentration and the like—conditions that are unlikely to be so stably maintained in natural settings:
As Bains (2020) notes, "the issue in OOL research is not just 'can we make it' but also 'can we stop making everything else'". (See, Supplementary Information, in Bains, W. (2020). Getting beyond the Toy Domain. Meditations on David Deamer’s “Assembling Life”.).
"In my view, almost all the OOL chemistry that I see is Toy Domain chemistry. It is making single types of biochemicals in a controlled laboratory setting using pure chemicals that might, just might, have been present in trace amounts in a complex mixture of thousands of other chemicals at OOL, under conditions that might have existed and might have persisted long enough, and then stopping the reaction at exactly the right time to maximize the yield of what you want... It neglects that many of the postulated starting materials are themselves unstable. It neglects that they will react with other chemicals present. It neglects that the intermediates will all react with each other, and with the products.
(The occasional comment that some unstable compound is present in meteorites/Titan/comets and so could have been present on early Earth as feedstock for abiotic chemistry is obviously absurd. Stability is determined by environmental conditions, including temperature and presence of other chemicals, including water. Presence of materials on Titan is no more relevant to the proposed inputs to terrestrial scenarios that the presence of DNA on Earth is precedence for life on Mercury. An unstable compound might be a reactive radical or other fleeting intermediate under terrestrial conditions, but that is a different argument.)
Of course OOL chemists understand that 99% pure reagents were not available at OOL."
Bains, W. (2020). Getting beyond the Toy Domain. Meditations on David Deamer’s “Assembling Life”.).
3. Third, simply having the 7 oligonucleotide pieces is not sufficient. Contrary to Richard's confusion, they will not “automatically assemble” into a 150 nucleotide “self-replicator”. They will sit and do nothing unless they are energetically activated, and not just any way. The 7 pieces have to be energetically activated in a very specific way to promote the correct reaction (and any reaction at all). The 7 pieces were activated with phosphorimidazolide (Imp). And how was this accomplished in the lab? Lo and behold it was with our old friend—“condensing agent” EDC (Ethylene Dichloride) (See, Supplementary Information, Wachowiusa & Hollinger 2019), which we’ve already established is not a prebiotically plausible chemical reagent:
“Coupling reactions were carried out using EDC, well known to be efficient for the phosphoramidate ligation of nucleotides. Though it does not represent a plausible reagent in a prebiotic environment.” (Liu, Z., Ajram, G., Rossi, J. C., & Pascal, R. (2019). The chemical likelihood of ribonucleotide-α-amino acid copolymers as players for early stages of evolution. Journal of molecular evolution, 87(2), 83-92.)
 |
| Fig. 1 Wachowiusa & Hollinger 2019 The RNA fragments must be energetically activated (red circle) in a specific way to promote bonding (using prebiotically implausible EDC); and additional RNA "splint" strands are needed to scaffold assembly |
4. But let’s say it somehow happens anyway without EDC, and we now have our 7 phosphorimidazolide (Imp) activated oligonucleotide pieces. Will the 7 pieces “automatically self-assemble” into our 150 nucleotide “self-replicator” now? Absolutely not (Perhaps, Richard should read his own sources more closely). Activation will increase the probability of bonding, but there are a myriad number of ways they can do so—only one of which is correct—and the 7 pieces must also connect in the precise order in order to make a 150 nucleotide RNA that actually functions. They’re not going to “automatically self-assemble” themselves in the correct order on their own. No, they only do so with the help of added helper “splints”. Specifically, six additional RNA oligonucleotide strands between 20-30 nucleotides long (See, Figure 1, and Supplementary Information, Wachowiusa & Hollinger 2019). These “splints” were specially engineered to not only physically orient the 7 strands in favorable positions to promote bonding between the energetically activated ends, but also to ensure the 7 pieces were correctly matched with their correct partner(s), so that all 7 strands connect to each other in the correct sequential order, which is critical to proper catalytic functioning.
 |
| Richard erroneously believes a self-replicator will magically assemble itself |
 |
| Energetically activated RNA nucleotides and additional RNA "splints" are required |
 |
| The seven RNA strands don't magically assemble themselves |
 |
| Six specially sequenced RNA "splints" are needed to scaffold and help assemble the seven fragments in the correct order, and even then it does not work 99.5% of the time |
 |
| Even when the fragments do connect, most of the time it still does not fold correctly into a functioning RNA replicase ribozyme |
5. So let’s take stock of the situation: In order to “automatically self-assemble” the 7 strands into a 150 nucleotide RNA molecule, we actually need thirteen RNA strands, not seven: we need the seven energetically activated pieces (6x20 nt, 1x30 nt) and “the addition of the corresponding six complementary RNA splints (5×20 nt, 1×30 nt),” for a total of 280 RNA nucleotides.
Let’s not miss that: we actually need thirteen 20-30 nt strands—not seven—totaling 280 nucleotides to make this 150 nucleotide RNA. (1 chance in 4^280 = 1 in 10^168).
6. But now we have a functioning 150 nt RNA “self-replicator,” right? Wrong. The complementary splints base-pair bonded to the seven pieces must now be removed so they don’t interfere with the functioning of the assembled 150nt RNA.
7. Thus, “on paper” we need 13 RNA strands totaling 280 nucleotides to spontaneously form in the same location around the same time to get our 150 nucleotide “self-replicator” “toy model”. And even if it does happen, so what? Without substrates to then act on in a catalytic reaction our 150nt RNA sits around and does nothing. But even if we have substrates, again, so what? The RNA Wachowiusa & Hollinger created is not actually a “self-replicator”. See, for example, Piette, B. M., & Heddle, J. G. (2020). A peptide–nucleic acid replicator origin for life. Trends in ecology & evolution, 35(5), 397-406, which discusses the Wachowiusa & Hollinger study:
"The simplest RNA World theory requires only a self-replicating ribozyme. This could be an RNA strand with ligase activity; that is, self-templating using pre-existing large fragments of complimentary sequences. Ligase (but not self-replicating ligase) ribozymes do exist in nature and in vitro designed/evolved ribozyme ligases have been produced, beginning with the work of Bartel and Szostak. Efforts have been made to produce minimal ligases, for example by Kurihara et al. when they made an ~50 nt functional version of R3C ligase, similar in length to the small L1 ligase. These ligases, however, do not self-replicate…[R]ecent work suggests that functional ribozyme ligases can be produced spontaneously (i.e., nonenzymatically) from short building blocks more likely to have been present on the early Earth [e.g., Wachowiusa & Holliger 2019]. However, again, these ligases do not replicate themselves. This may be an insurmountable problem, as Wachowius et al. stated: ‘Fundamentally, emergence of new functions when assembling long sequences is confounded by the nature of such activities: ligases use less information to choose substrates than is required to define the ligase activity itself, so cannot copy themselves (or other components) from sequences lacking that information, i.e. random sequence’.” (emphasis added)
Thus, Richard's 150 nt RNA is not even a “self-replicator”!
8. But there are more problems still. We still run into "The 'Concentration Threshold' Problem." Thirteen 20-30 nt RNA strands is not sufficient to make our 150nt RNA. Maybe it’s enough “on paper” in “toy model” land, but it’s nowhere near enough in the real-world. Realistically, we need hundreds of thousands to millions, billions, or even trillions of identical copies of these 13 strands to overcome the concentration threshold and initiate a chemical reaction to make hundreds of thousands to millions of 150nt RNA molecules to overcome a second concentration threshold to initiate a catalytic reaction that isn’t even “self-replication”.
9. But if somehow against all odds we do get a spontaneous pool filled with hundreds of thousands to millions of identical copies of our 13 strands to overcome our initial concentration threshold (ignoring the synthesis of the 13 strands to begin with, that is), then at least we’re guaranteed to make the same number of copies of our 150nt RNA, right? Wrong again. Even starting with pure reagents and trillions of copies of our 13 strands, Richard's laughably so-called “automatic self-assembly” gives a paltry yield of 0.5%. Only 0.5% of all those trillions of strands “automatically self-assemble” into our full-length 150nt RNA, as Wachowiusa & Holliger explain:
“We provide a proof-of-principle demonstration for the spontaneous assembly of a functional 150 nt RNA polymerase ribozyme…from up to 7 short (20–30 nt), non-functional 5’-2-methylimidazole-activated RNA oligonucleotides via splint assisted non-enzymatic ligation in a “one pot” reaction. The final yield of the full-length ribozyme from 7 pieces is currently relatively modest (0.5 %), which is likely related to the chemical instability and short half-life of the phosphorimidazole activating groups in aqueous solution, the incomplete formation of ligation junctions due to RNA misfolding and incomplete ligation of assembled junctions.”
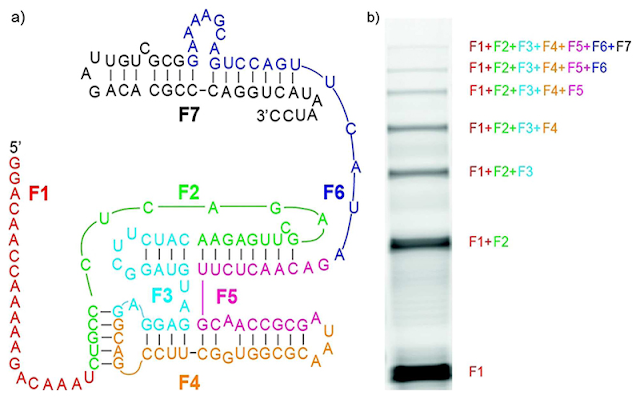 |
| Fig. 4 Wachowiusa & Hollinger 2019 The completed 150 nt non-self-replicating RNA replicase ribozyme (Fig. 4a). The thickness of the gel bands on the right (Fig. 4b) correspond to yield. The thicker and darker the black gel band, the higher the yield. Notice that the top band for the completed assembly of all seven strands is very faint, and corresponds to a paltry 0.5% yield. |
Did you catch that? In addition to the problems of RNA misfolding causing “incomplete formation of ligation junctions” and “incomplete ligation of assembled junctions” (that is, even the splint-assisted junctions didn’t always result in correct “chaining” of the RNA strands), the low yield was also “likely related to the chemical instability and short half-life of the phosphorimidazole activating groups in aqueous solution.” In other words, not only do we have to energetically activate the RNA strands—which is problematic in itself—but even if we accomplish this feat our “success” is short-lived. These activated RNAs don’t stay activated forever or even for very long, but are quickly hydrolyzed even in controlled laboratory settings! (There is a reason for this: molecules must typically be energetically activated in order to become reactive, but the fact that they are more reactive makes them unstable as a result).
10. The low 0.5% yield (which to organic chemists is considered a “relatively modest” yield—that speaks volumes in itself), in turn, means that we don’t have enough copies of our full-length 150 nt non-self-replicator to meet our second concentration threshold to initiate RNA catalysis. This is why Wachowiusa & Holliger then had to amplify the low yield with PCR to meet the concentration threshold to ‘prove’ that we can still ‘spontaneously’ get catalytic function.
11. And that’s not even the half of it. How deep do we want to keep going down the rabbit hole? To this we can add the optimized reaction conditions for both the assembly of the 7 strands and the optimal subsequent catalytic function: eutectic ice at -7 C. But “Non-enzymatic polymerisation of RNA oligomers from 5’-phosphorimidazole-activated monomers in ice generates preferentially short oligomers with maximum lengths of ~20 nt with a mixed base composition.”
But wait! Why not just use montmorillonite clay catalysis then—which Totani uses to model RNA polymerization for his calculations—since it’s far easier than eutectic ice to synthesize longer RNA polymer strands this way and also supposedly “solves” the homochirality problem, too? Well, as Wachowiusa & Holliger explain, “On montmorillonite clay longer oligomers have been observed but only for low complexity sequences.” What makes them “low complexity”? Answer: They are homopolymers consisting of a single type of nucleobase. (See, e.g., Huang, W., & Ferris, J. P. (2006). One-step, regioselective synthesis of up to 50-mers of RNA oligomers by montmorillonite catalysis. Journal of the American Chemical Society, 128(27), 8914-8919. The 50-mer RNA oligomers they made were homopolymers of repeating nucleobases like oligo(A)’s or oligo(U)’s: “UUUUUUUUUUU….etc.”
And this highlights another problem: Richard indiscriminately generalizes experimental results for specific contexts; erroneously treating them like they apply to every situation. Thus, Totani, similarly is using simplified “toy models” as the basis for his calculations, including using homopolymer montmorillonite clay catalysis as his polymerization model. Specifically, Totani uses the Kawamura & Ferris (1994) “experiment on clay surfaces as a model case” for “RNA polymerization by randomly adding activated monomers to an oligomer as a Poisson process.” (See, Totani’s reference 18). But not “random” in reality, because Kawamura & Ferris only made oligo(A)’s—strands of adenosine nucleotides “AAAAAAAAAAAA…etc.”
So, does Richard think we can get a functional RNA “self-replicator” from a single type of nucleobase?
12. And then there’s the problem of the eutectic ice conditions itself, which do not fit with Richard's “Creation and evolution of impact-generated reduced atmospheres of early Earth” scenario, as that article notes itself (Zahnle et al. (2020). Creation and evolution of impact-generated reduced atmospheres of early Earth. The Planetary Science Journal, 1(1), 11.):
“A possible problem posed by the biggest [Moneta] impact is that, although it brings the most reducing power, it is also the most likely to leave the surface too hot to promote prebiotic evolution and for a long time. Even ignoring CO2 and CH4, the greenhouse effect provided by tens of bars of H2 could raise the surface temperature to 400 K or more. Adding CO2 or CH4 or other greenhouse gases would make the surface still hotter. Impacts that are 10- to 100-fold smaller may therefore seem preferable, as these are more likely to leave the surface in a temperate state, albeit the reducing conditions do not last as long as for the bigger events.”
And if that’s problematic for prebiotic syntheses in general, then how much more so for scenarios that require eutectic ice?
13. But we don’t even have to go that big. In order to amplify the low 0.5% yield, Wachowiusa & Hollinger used laboratory PCR, as already noted. But PCR amplifies via numerous repeated annealing-extension (heating-cooling) cycles where RNA strands are copied via template synthesis, but then have to be heated to break hydrogen bonds so the complementary base-paired parent and daughter RNA strands can dissociate from each other. These repeated heating-cooling cycles typically range between 48-72 C; well above our -7 C eutectic ice conditions even during the PCR “cooling” phases.
In other words, so much for Richard's claim that all this can happen in “natural conducive environments”. The Wachowiusa & Hollinger’s lab protocol required them to go from -7 C eutectic ice to assemble the 150nt RNA, then 48-72 C for PCR amplification, then back to eutectic ice for optimal RNA catalysis function.
14. And even if we solve all this, how on earth are we going to “transition” from ice to the ambient temperatures needed for life as we know it? How are we going to “evolve” that? What is Richard's non-speculative, empirically established scenario?
15. And then there’s….And the rabbit hole goes on and on, deeper and deeper.
And once again, even if all this happens, this is not a self-replicating RNA!



Comments
Post a Comment